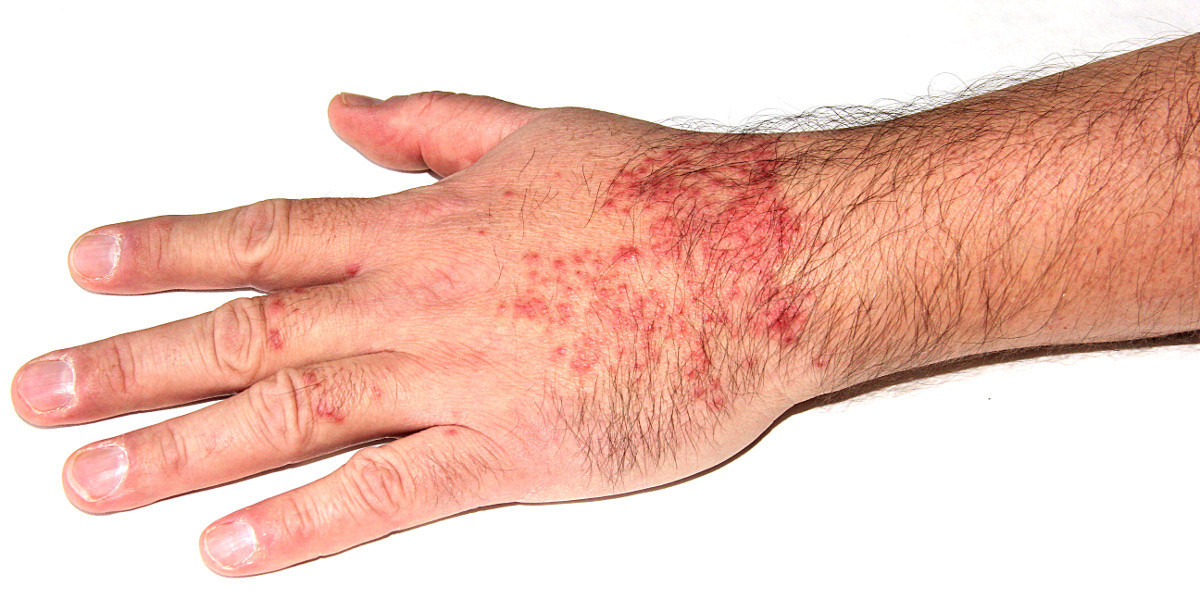
Eczema, medically known as atopic dermatitis (AD), is a persistent, inflammatory skin disorder that affects millions globally. Patients often face daily challenges such as intense itching, red patches, and dry, scaly skin that disrupt their quality of life. Recent advancements in topical treatments have paved the way for innovative therapies that target the disease’s underlying inflammatory processes. Among these breakthroughs, OPZELURA (ruxolitinib) cream has emerged as a beacon of hope for many, boasting rapid action and a favorable safety profile that distinguishes it from traditional therapies.
For more in-depth insights on OPZELURA’s development and future potential, download the full report @ OPZELURA Market Report
Understanding Atopic Dermatitis
Atopic dermatitis is a multifactorial condition arising from a complex interplay of genetic predispositions, environmental triggers, and immune system dysregulation. It is most common among children, although many individuals continue to experience symptoms well into adulthood. Characteristic symptoms include dry, scaly skin, persistent itching, and inflamed patches that can sometimes crack or ooze. The overactive immune response in AD leads to chronic inflammation and disrupts the skin barrier, increasing vulnerability to infections.
Conventional treatments have ranged from emollients and corticosteroids to calcineurin inhibitors and systemic immunosuppressants. However, long-term use of some treatments, particularly corticosteroids, can lead to adverse effects such as skin thinning or hormonal imbalances. This reality underscores the pressing need for alternative therapies that provide effective, sustained relief without the long-term complications seen with traditional options.
Introduction to OPZELURA (Ruxolitinib)
OPZELURA represents a significant stride forward in the management of atopic dermatitis. Developed by Incyte Corporation, this topical formulation harnesses the power of ruxolitinib, a potent Janus kinase (JAK) inhibitor. Approved by the U.S. Food and Drug Administration (FDA) in September 2021 for patients aged 12 and older who are not immunocompromised, OPZELURA offers a non-steroidal approach to treating AD.
The introduction of OPZELURA has been met with enthusiasm from both the medical community and patients, a sentiment reflected in its growing market presence. Notably, OPZELURA sales have surged since its launch, signaling strong market demand and acceptance among healthcare providers. Its targeted approach provides a much-needed alternative for individuals who either cannot tolerate or do not respond adequately to traditional steroid-based treatments.
For more detailed insights and the latest updates on OPZELURA, visit the OPZELURA Market update
Mechanism of Action (MOA)
The efficacy of OPZELURA lies in its precise targeting of the inflammatory pathways that drive atopic dermatitis. The OPZELURA active ingredient, ruxolitinib, functions by inhibiting JAK1 and JAK2 pathways—key players in the JAK-STAT signaling cascade. This pathway is critical for transmitting signals from cytokines and growth factors that regulate the immune system.
By selectively blocking these pathways, OPZELURA effectively reduces inflammation, mitigates intense itching, and restores the skin barrier’s functionality. This targeted suppression of the immune response translates to a rapid alleviation of symptoms, often noticeable within 24 to 48 hours of application. Understanding OPZELURA’s Mechanism of Action is essential, as it highlights the cream’s unique capacity to deliver relief without the systemic side effects typically associated with oral JAK inhibitors or prolonged steroid use.
Clinical Efficacy of OPZELURA
The clinical success of OPZELURA is well-documented through rigorous OPZELURA Clinical Trials. Two pivotal phase 3 trials, TRuE-AD1 and TRuE-AD2, enrolled over 1,200 patients with mild to moderate AD. These trials provided compelling evidence of OPZELURA’s rapid action and efficacy. Key findings from these studies include:
- A significant proportion of patients achieved clear or nearly clear skin as measured by the Investigator’s Global Assessment (IGA) score.
- Many patients experienced a rapid reduction in itch severity, with improvements reported as early as 24 to 48 hours following the first application.
- Marked improvements were also observed in the eczema area and severity index (EASI) scores, underscoring the cream’s ability to reduce overall disease burden.
- The treatment was well tolerated, with minimal systemic absorption, thereby reducing the risk of broader systemic side effects.
In addition to these clinical findings, real-world evidence supports the effectiveness of OPZELURA. Patients consistently report sustained symptom relief, improved sleep quality, and overall enhanced quality of life, which in turn has positively influenced OPZELURA sales. This clinical success, paired with a strong safety profile, has paved the way for rapid adoption in dermatological practice.
For further insights and detailed research on this breakthrough treatment, visit OPZELURA insights
Safety Profile and Side Effects
OPZELURA’s safety profile is another reason for its growing popularity among both clinicians and patients. As a topical JAK inhibitor, it offers significant advantages over systemic treatments, particularly in terms of minimizing systemic exposure. The most commonly reported side effects include:
- Mild application site reactions, such as redness, burning, or stinging
- Nasopharyngitis, which resembles common cold symptoms
- Headaches
While theoretical concerns exist regarding systemic effects—such as the risk of infections, blood clots, or changes in blood counts—clinical data indicates that these risks are substantially mitigated by the cream’s low systemic absorption. This favorable safety profile ensures that OPZELURA remains a viable option for long-term management of AD, particularly when compared to the prolonged use of corticosteroids or other immunomodulators.
Comparisons with Other Treatments
When evaluating treatment options for atopic dermatitis, it is important to consider how OPZELURA compares with traditional therapies:
- Topical Corticosteroids: While corticosteroids are effective at reducing inflammation, their long-term use can lead to skin thinning and hormonal imbalances. In contrast, OPZELURA offers similar or superior efficacy without these adverse effects.
- Calcineurin Inhibitors: Agents like tacrolimus and pimecrolimus have been used off-label for AD but are often associated with burning sensations upon application. OPZELURA, on the other hand, is designed for a more tolerable application experience.
- Systemic Therapies: Systemic treatments such as dupilumab or oral JAK inhibitors are typically reserved for moderate to severe cases due to their broader side effect profiles. OPZELURA’s localized, topical application makes it ideal for treating localized AD while minimizing systemic risks.
These comparisons underline OPZELURA’s role as a robust alternative that combines rapid efficacy with a reduced risk of long-term complications.
For additional insights on OPZELURA’s transformative potential, please download the full OPZELURA report
Patient Considerations
OPZELURA is particularly suited for patients with mild to moderate atopic dermatitis who have not found sufficient relief with conventional therapies. Ideal candidates include:
- Individuals experiencing frequent flare-ups who require consistent, long-term management without the complications associated with steroids.
- Patients who prefer a non-steroidal option that provides quick relief from itching and inflammation.
- Those aged 12 and older who are not immunocompromised, as stipulated in the FDA OPZELURA Approvals.
However, certain patients should exercise caution or avoid OPZELURA altogether. These include individuals with active infections or those with a history of serious cardiovascular or thromboembolic conditions. Pregnant or breastfeeding women should consult with their healthcare provider before starting treatment.
Future Perspectives
The successful launch and adoption of OPZELURA have opened new avenues in the treatment of not only atopic dermatitis but also other inflammatory skin conditions. Ongoing research is investigating the broader applications of JAK inhibitors in dermatology, including potential uses in conditions such as vitiligo, psoriasis, and alopecia areata. Researchers continue to explore ways to optimize OPZELURA’s Mechanism of Action for improved safety and efficacy in a wider range of patient populations. As our understanding of the molecular pathways underlying inflammatory skin disorders expands, treatments like OPZELURA are poised to become central to a more personalized and targeted approach to dermatologic care.
For those looking to explore more about this breakthrough treatment, download the full OPZELURA Insights Report
Conclusion
OPZELURA (ruxolitinib) cream stands at the forefront of a new era in atopic dermatitis treatment. By addressing the root causes of inflammation through its targeted inhibition of JAK pathways, it provides rapid symptom relief and improved quality of life for eczema sufferers. With a robust body of evidence from OPZELURA Clinical Trials, a favorable safety profile, and growing OPZELURA sales underscoring its market acceptance, this topical therapy offers a much-needed alternative to traditional treatments. As ongoing research continues to explore further applications and optimizations, OPZELURA is set to redefine the standard of care for inflammatory skin disorders, offering lasting hope to millions affected by eczema.
Related Reports
Atopic Dermatitis Market Insight, Epidemiology and Market Forecast
Radiation Dermatitis Market Insight, Epidemiology and Market Forecast
Seborrhoeic Dermatitis - Market Insight, Epidemiology and Market Forecast
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve their performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.