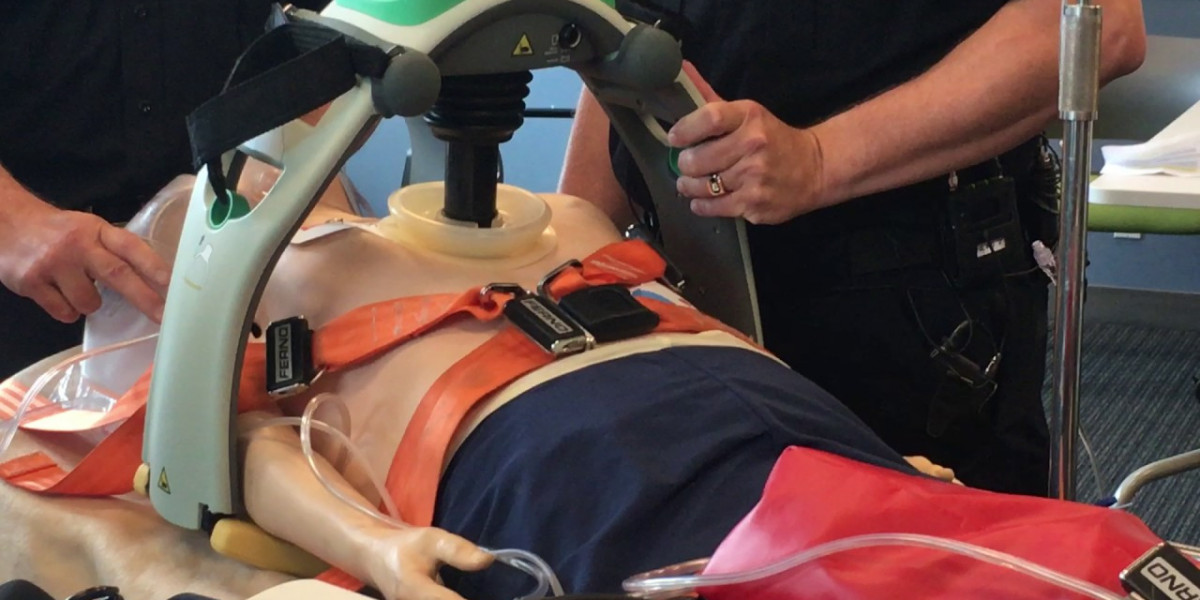
The Automated CPR Devices Market has seen significant growth in recent years, driven by advancements in technology and an increasing emphasis on improving patient outcomes during medical emergencies such as cardiac arrest. Despite this, there are several restraints that may limit the market’s growth and adoption. These factors include economic constraints, technological limitations, regulatory hurdles, and challenges in healthcare infrastructure. Below, we explore these factors in detail to understand the challenges facing the automated CPR devices market.
Economic Constraints
One of the primary restraints in the automated CPR devices market is the high cost associated with the acquisition and maintenance of these devices. Automated CPR devices, especially those that are equipped with advanced features such as real-time monitoring, data analytics, and integration with hospital systems, come with a hefty price tag. This makes it difficult for healthcare institutions, particularly those in developing countries, to justify the investment. In regions with limited healthcare budgets, where there are already competing priorities, automated CPR devices may be viewed as an expensive luxury rather than an essential tool. As a result, the market penetration of these devices is slower in low- and middle-income regions, which can hinder global growth.
Additionally, healthcare systems with financial constraints may also struggle with the recurring costs of maintenance, calibration, and replacement of parts, which are essential to ensure the devices function correctly. Without the proper support infrastructure, automated CPR devices may face underutilization or operational inefficiencies.
Technological Limitations
Despite advancements in the field of automated CPR, the technology still faces several limitations. While these devices are designed to deliver consistent chest compressions during a cardiac arrest, they are not without technical shortcomings. One such limitation is the device's ability to provide adequate chest compression depth and rate in all patients, especially those with varying body sizes, shapes, or conditions. Although automated devices have been designed to adjust to some extent, the variability in patients’ anatomy can impact the effectiveness of the device. This limitation is a major concern, especially in emergencies when every second counts.
Furthermore, there are limitations in the ability of automated CPR devices to adapt to complex or variable clinical conditions. Cardiac arrest scenarios can differ greatly depending on the underlying cause of the event, the patient's health status, and other critical factors. Automated CPR devices may not always be able to adjust in real-time to these variations, limiting their effectiveness in certain cases.
Regulatory Challenges
Regulation is a significant barrier to the rapid adoption of automated CPR devices. These devices are considered medical equipment and, therefore, need to undergo rigorous approval processes from regulatory authorities such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The approval process for medical devices can be time-consuming, requiring substantial investment in research, clinical trials, and documentation to demonstrate safety and efficacy.
Moreover, regulatory requirements for automated CPR devices vary across different regions, further complicating the market landscape. For example, in some regions, stricter regulations may demand more extensive clinical trials or longer timelines for approval. In addition, international trade barriers, such as tariffs and regulatory differences, can make it difficult for companies to expand their markets globally, stalling the widespread distribution of these devices.
Lack of Training and Awareness
Another significant restraint in the automated CPR devices market is the lack of proper training and awareness among healthcare professionals and emergency responders. Although automated CPR devices are designed to be user-friendly, they still require trained personnel to operate them effectively. In many settings, especially in remote or underdeveloped regions, there may be a lack of adequate training programs for emergency responders or healthcare workers.
Moreover, healthcare facilities that adopt automated CPR devices may not have the resources to continuously train their staff, leading to inconsistent use and potentially ineffective outcomes during emergencies. This could ultimately undermine the perceived value of the device, as healthcare professionals may become reluctant to rely on it in life-threatening situations. Without widespread and ongoing education, automated CPR devices may not be fully integrated into standard emergency care protocols, limiting their impact.
Ethical Concerns
The growing reliance on technology in healthcare, particularly in life-or-death situations, raises ethical concerns that could also act as a restraint on the market. Some critics argue that the automation of critical medical interventions, such as CPR, could diminish the role of human judgment and intervention. In certain cases, healthcare professionals may question whether automated CPR devices could override the nuances of clinical decision-making that come with hands-on care.
Furthermore, there are concerns about the potential loss of human empathy and interaction in emergency care scenarios. While automated devices can save lives by providing timely and effective chest compressions, the emotional and psychological aspects of patient care are also important, especially when dealing with families and loved ones during traumatic events. The risk of reducing human interaction and support may contribute to resistance against automated CPR devices in some healthcare settings.
Conclusion
While the automated CPR devices market holds significant promise, it faces a range of restraints that could impact its growth and widespread adoption. High costs, technological limitations, regulatory hurdles, and the lack of adequate training and awareness are among the most pressing issues. Furthermore, ethical concerns surrounding the automation of life-saving procedures may also contribute to resistance against these devices. However, as technology continues to advance and healthcare systems evolve, some of these challenges may be mitigated, leading to greater integration of automated CPR devices into emergency care protocols worldwide.
read more:.
| https://www.pristinemarketinsights.com/automated-cpr-devices-market-report |