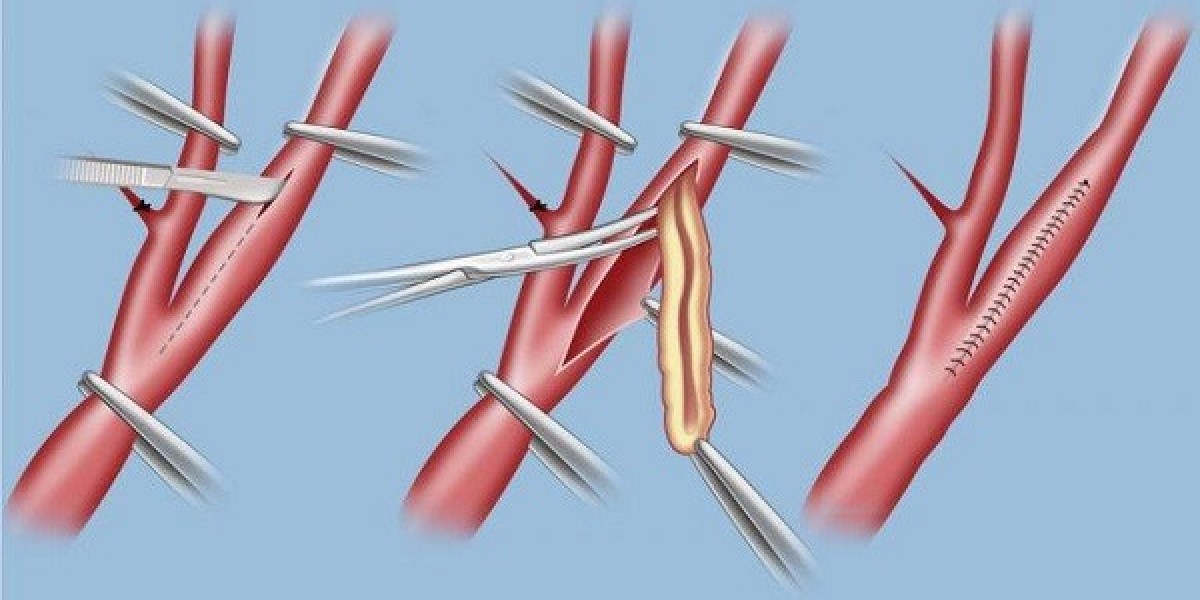
The vascular patches market is witnessing notable growth due to rising cardiovascular disease prevalence and technological advancements. However, several key restraints challenge its expansion and adoption across healthcare settings. Understanding these constraints is crucial for manufacturers, healthcare providers, and investors to navigate the market effectively and develop strategies to overcome them.
High Cost of Advanced Vascular Patches
One of the most significant restraints to the vascular patches market growth is the high cost associated with advanced patch materials and technologies. Biologic patches, hybrid materials, and drug-eluting vascular patches offer improved clinical benefits but come with higher price points compared to conventional synthetic options.
These elevated costs limit accessibility, especially in developing and underdeveloped regions where healthcare budgets are constrained. Even in developed countries, the increasing financial burden of cardiovascular surgeries and associated devices can restrict widespread adoption, particularly in outpatient or resource-limited settings.
Cost sensitivity also impacts purchasing decisions by hospitals and surgical centers, which must balance efficacy, safety, and budget considerations. Without cost-effective alternatives or reimbursement support, market penetration of premium vascular patches remains a challenge.
Stringent Regulatory Environment
The vascular patches market is subject to a rigorous regulatory landscape designed to ensure patient safety and device efficacy. While necessary, these regulatory requirements can act as a restraint by prolonging product development timelines and increasing associated costs.
Regulatory bodies such as the U.S. FDA, European Medicines Agency (EMA), and others require extensive preclinical and clinical data to approve new vascular patches, especially those incorporating novel materials or drug delivery systems. The lengthy approval process can delay market entry, reduce return on investment, and deter smaller companies from innovating.
Additionally, differences in regulatory frameworks across regions complicate global commercialization strategies. Manufacturers must navigate variable standards, documentation requirements, and post-market surveillance obligations, adding complexity and expense.
Limited Clinical Awareness and Training
Another barrier restraining the vascular patches market is limited awareness and expertise among healthcare professionals regarding new vascular patch technologies and applications. Surgeons accustomed to traditional materials and techniques may hesitate to adopt novel products without sufficient clinical evidence and training.
The learning curve associated with minimally invasive procedures and handling advanced patches also affects surgeon confidence and utilization rates. Without comprehensive education programs and clinical demonstrations, adoption can remain slow, especially in less-developed healthcare settings.
Moreover, the lack of standardized guidelines for vascular patch selection and use contributes to inconsistent clinical practices, further limiting market growth potential.
Material Limitations and Biocompatibility Concerns
Although innovations in patch materials have improved outcomes, certain material-related challenges continue to restrain market expansion. Synthetic patches such as ePTFE and polyester, while durable, may cause inflammatory responses, scarring, or thrombosis in some patients, leading to complications.
Biologic patches, despite better biocompatibility, can vary in quality depending on the source and processing methods, sometimes triggering immune reactions or infections. Ensuring consistent performance and safety across different batches remains a challenge.
Hybrid patches and drug-eluting variants introduce additional complexity, as integrating multiple materials or pharmacological agents can increase risks related to degradation, toxicity, or unexpected interactions. These uncertainties impact surgeon preference and regulatory scrutiny.
Risk of Post-Surgical Complications
Post-operative complications such as infection, thrombosis, patch failure, and graft rejection continue to restrain vascular patch adoption. Although advancements have reduced these risks, no vascular patch is entirely free from potential adverse effects.
These complications not only affect patient outcomes but also contribute to higher healthcare costs due to reoperations or extended hospital stays. Concerns about long-term durability and patch integrity influence clinical decision-making and cautious use of new products.
Manufacturers must continually invest in improving patch safety and provide robust clinical data to address these challenges effectively.
Competitive Alternatives and Market Fragmentation
The vascular patches market faces competition from alternative treatment options and technologies that can act as restraints. For example, autologous vein grafts remain a preferred choice in many vascular surgeries due to excellent biocompatibility and long-term outcomes, reducing demand for synthetic or biologic patches.
Additionally, advances in stent technology, angioplasty, and other minimally invasive interventions offer non-patch solutions for certain vascular conditions. These alternatives can limit market growth by providing less invasive or more cost-effective options.
The market is also fragmented with numerous small and large players, making it challenging for companies to establish dominance without significant investment in research, marketing, and partnerships.
Supply Chain and Manufacturing Challenges
Complexity in the manufacturing process and supply chain logistics can also restrain the vascular patches market. Producing high-quality biologic and hybrid patches requires stringent quality control, specialized facilities, and reliable sourcing of raw materials.
Disruptions in supply chains, such as those caused by geopolitical issues or pandemics, can impact availability and pricing. Smaller companies may face difficulty scaling production to meet increasing demand, while larger firms must manage costs carefully to remain competitive.
Economic and Healthcare Disparities
Economic disparities and unequal healthcare access worldwide limit vascular patch market potential in low- and middle-income countries. In many regions, lack of infrastructure, limited surgical expertise, and prioritization of primary healthcare needs overshadow investment in advanced vascular repair technologies.
Insurance coverage and reimbursement policies vary widely, affecting affordability and hospital procurement decisions. Until these disparities are addressed, market growth will be concentrated primarily in high-income countries, restraining global expansion.
Conclusion
While the vascular patches market is growing due to rising cardiovascular disease incidence and technological progress, several restraints impact its pace and breadth of adoption. High costs, stringent regulatory hurdles, limited clinical awareness, material limitations, and competition from alternatives all pose challenges.
Addressing these restraints through cost reduction strategies, streamlined regulatory pathways, enhanced surgeon training, and continuous product innovation will be essential to unlock the full potential of the vascular patches market. For manufacturers and healthcare providers, understanding and navigating these challenges is key to ensuring better patient outcomes and sustainable market growth in the future.